Dipole Meaning In Chemistry ~ 3 3k views view 2 upvoters. In chemistry dipole moments are applied to the distribution of electrons between two bonded atoms. This arrow symbolizes the shift of electron density in the molecule. Dipole dipole london dispersion also known as van der waals interactions hydrogen bonding and ionic bonds are the main types of intermolecular interactions responsible for the physical properties of compounds. Those atoms that are more electronegative pull the bonded electrons closer to themselves. The existence of a dipole moment is the difference between polar and nonpolar bonds. Dipoles generally occur between two nonmetals that share electrons as part of their bond. A permanent magnet such as a bar magnet owes its magnetism to the intrinsic magnetic dipole moment of the electron. Strong colors indicate highest and lowest potential where the opposing charges of the dipole are located. Contour plot of the electrostatic potential of a horizontally oriented electrical dipole of infinitesimal size. Indeed lately has been hunted by users around us, perhaps one of you. People now are accustomed to using the internet in gadgets to see image and video information for inspiration, and according to the name of this post I will discuss about Dipole Meaning In Chemistry. A dipole is a separation of electrical charges. A permanent magnet such as a bar magnet owes its magnetism to the intrinsic magnetic dipole moment of the electron. This arrow symbolizes the shift of electron density in the molecule. In chemistry the dipole moment is represented by a slight variation of the arrow symbol. Those atoms that are more electronegative pull the bonded electrons closer to themselves. In chemistry a dipole usually refers to the separation of charges within a molecule between two covalently bonded atoms or atoms that share an ionic bond. Noun in chemistry a permanent dipole describes the partial charge separation that can occur within a molecule along the bond that forms between two different atoms. It is denoted by a cross on the positive center and arrowhead on the negative center. If the net dipole moment is zero or very very small the bond and molecule are considered to be nonpolar. Molecules with a net dipole moment are polar molecules.
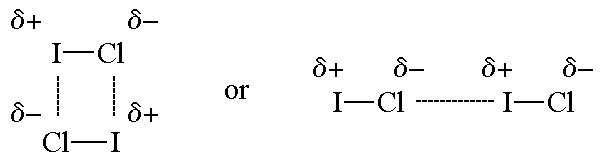
This arrow symbolizes the shift of electron density in the molecule. Dipoles generally occur between two nonmetals that share electrons as part of their bond. Dipole dipole london dispersion also known as van der waals interactions hydrogen bonding and ionic bonds are the main types of intermolecular interactions responsible for the physical properties of compounds.
Contour plot of the electrostatic potential of a horizontally oriented electrical dipole of infinitesimal size.
The attractive forces between the positive end of one molecule with the negative end of other molecule are called dipole dipole forces. Those atoms that are more electronegative pull the bonded electrons closer to themselves. Permanent dipole dipole is the 2 nd strongest intermolecular force with hydrogen bonds being the strongest and van der waals being the weakest. Dipole dipole london dispersion also known as van der waals interactions hydrogen bonding and ionic bonds are the main types of intermolecular interactions responsible for the physical properties of compounds.